Informations about Cefoperazone side effects:
For staff contact dermatitis from cephalosporins allergy was reported in a nurse to provide solutions cephalosporin administration to patients. Dermatitis resolve sister stopped training solutions. Hypersensitivity reactions of cefoperazone, often manifested as skin rashes, are common with cefoperazone and may require drug discontinuation. Drug fever and changes in Coombs' tests have also been reported. Allergic cross-reactivity may occur in patients allergic to penicillin. Cephalosporin class antibiotics have been associated with anaphylaxis, Stevens-Johnson syndrome, erythema multiforme, and toxic epidermal necrolysis.
Pokazywanie postów oznaczonych etykietą cefoperazone. Pokaż wszystkie posty
Pokazywanie postów oznaczonych etykietą cefoperazone. Pokaż wszystkie posty
piątek, 8 stycznia 2010
Dosage of Cefoperazone.
Usual dosage of Cefoperazone antibiotic:
The usual adult daily dose CEFOBID (sterile cefoperazone) is 2 to 4 g / day in equally divided doses every 12 hours. In severe infections or infections that are caused by less sensitive organisms, the daily dose and / or increased frequency. The patients were successfully treated with a total daily divided dose of 6-12 g in 2, 3 or 4 handle 1.5 to 4 grams per dose. Solutions CEFOBID and aminoglycoside should not be mixed directly, because it is a physical incompatibility between them. If combination therapy with aminoglycosides and CEFOBID this should be achieved sequential intermittent intravenous infusion, provided that separate secondary intravenous tubing is used, and that the main intravenous tubing properly irrigated with an approved solvent between doses.
Dosage of cefoperazone in injections looks different.
The usual adult daily dose CEFOBID (sterile cefoperazone) is 2 to 4 g / day in equally divided doses every 12 hours. In severe infections or infections that are caused by less sensitive organisms, the daily dose and / or increased frequency. The patients were successfully treated with a total daily divided dose of 6-12 g in 2, 3 or 4 handle 1.5 to 4 grams per dose. Solutions CEFOBID and aminoglycoside should not be mixed directly, because it is a physical incompatibility between them. If combination therapy with aminoglycosides and CEFOBID this should be achieved sequential intermittent intravenous infusion, provided that separate secondary intravenous tubing is used, and that the main intravenous tubing properly irrigated with an approved solvent between doses.
Dosage of cefoperazone in injections looks different.
Etykiety:
antibiotics,
cefoperazone,
cephalosporin antibiotics,
health
Cefoperazone and Sulbactam for injection.
Cefoperazone and sulbactam injections are indicated for the treatment of the following infections caused by bacterias sensitive for cefoperazone and sulbactam:
1. Infections of upper and lower respiratory systems.
2. Upper and lower urinary tract infection
3. Peritonitis, cholecystitis, cholangeitis and other intra-abdominal infections.
4. Ichoremia
5. Cephalomeningitis
6. Skin and soft tissue infections
7. Bone and joint infections
8. Pelvic inflammation, endometritis, gonorrhea and other infections of genital system.
1. Infections of upper and lower respiratory systems.
2. Upper and lower urinary tract infection
3. Peritonitis, cholecystitis, cholangeitis and other intra-abdominal infections.
4. Ichoremia
5. Cephalomeningitis
6. Skin and soft tissue infections
7. Bone and joint infections
8. Pelvic inflammation, endometritis, gonorrhea and other infections of genital system.
Etykiety:
antibiotics,
cefoperazone,
cephalosporin antibiotics,
health
Cefoperazone Cephalosporin Antibiotic
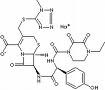
Chemistry of cefoperazone - cefoperazone is a third generation cephalosporin, cefoperazone sodium with piperazine side chain, there antipseudomonal activity. It comes as a white crystalline powder and is readily soluble in water and sparingly soluble in alcohol. At room temperature, temperature, cefoperazone sodium, which has a maximum solubility in compatible IV solution 475 mg / ml (at a concentration of 333 mg / ml, intense and prolonged shaking may) be required. Cefoperazone dissolved have a pH of 4.5 to 6.5. Each gram contains 1.5 mmol sodium.
Storage / Stability / Compatibility of Cefoperazone
Sterile Powder for Injection should be stored at a temperature below 25 ° C and protected from light. After the resolution is not well protected from light. After the dissolution of cefoperazone sodium is generally stable at room temperature for 24 hours up to 5 days if refrigerated at different IV solutions (eg sterile or bacteriostatic water for injection, dextrose in water / salt / LRS solution Ringer's lactate injections, and IV saline Normasol R). Cefoperazone when frozen to -2 to -10 ° C in dextrose, salt, or sterile water for injection, is Cefoperazone sodium for 3 weeks (dextrose solution) for 5 weeks stable (water or saline solution).
Cefoperazone sodium is reported to be in line with cimetidine HCl, clindamycin phosphate, furosemide, and heparin sodium, acyclovir sodium, cyclophosphamide, esmolol HCl, famotidine, hydromorphone HCl, magnesium sulfate and morphine sulfate. This is allegedly in violation of some TPN mixture, doxapram HCl, gentamicin sulfate, Hetastarch, labetolol HCl, meperidine HCl, odansetron HCl, perphenazine, promethazine and sargostim.
Etykiety:
antibiotics,
cefoperazone,
cephalosporin antibiotics
Subskrybuj:
Posty (Atom)